Page researched and written by: Rachel Wu, Abbey Sayers, Rachel Baer, MSc; Reviewed by Andy McCarty, MS, LCGC
Prenatal and Fetal Genetic Testing
While Clover Genetics does not currently see actively pregnant patients due to the high in-person, potentially rapid, needs of these individuals. However, we do work with patients in the preconception stages or after the birth of their child to support any genetic counseling needs for the family, including Psychiatric Genetic Counseling. Regardless, it is important for our community to understand the different tests that may be offered or requested before, during, or after pregnancy. This includes those who have experienced pregnancy loss who want to identify possible genetic contributors to their losses.
Page Contents:
Preconception Carrier Screening
Prenatal (Antenatal: During Pregnancy) Screening and Testing
NIPT/NIPS
Amniocentesis
Chorionic Villus Sampling (CVS)
Products of Conception
Preconception Carrier Screening:
Screening for genetic disorders during the preconception stage before pregnancy can be a useful tool to support family planning. Carrier screening is the process of screening an individual for autosomal recessive or X-linked genetic conditions associated with changes to a single gene. The test is most commonly performed with a saliva sample, but a buccal swab of the cheek or a blood draw may also be used (8). The American College of Medical Genetics and Genomics (ACMG) and American College of Obstetricians and Gynecologists (ACOG) are two national organizations that define clinical guidelines for preconception genetic screening and testing. The ACMG performs genetic research and uses the research to develop evidence-based guidelines which help clinicians make decisions regarding genetic testing.
When carrier screening first became available about 50 years ago, screening was mainly performed for conditions that are more prevalent in people belonging to certain ethnic groups. For example, screening for Tay-Sachs Disease is highly recommended in individuals of Ashkenazi Jewish descent. In 2008, screening for Cystic Fibrosis and Spinal Muscular Atrophy began to be recommended by the ACMG for anyone regardless of ethnicity (9). Both of these conditions can have a neonatal or early childhood onset as well as typically severe presentation of symptoms that require specified care or treatments as early as possible, which are reasons why these disorders were added to ACMG screening recommendations (14).
As of 2023, the cost and amount of time it takes to sequence a gene has decreased significantly, making genetic testing more accessible. Now that this screening can reasonably be offered to those considering pregnancy across the United States, the ACMG recommends expanded carrier screening to most people. These panels scan for a greater number of disorders simultaneously. The ACMG does not define exactly which genes and disorders should be screened for in these expanded panels, so laboratories vary slightly in their available screening options, with panels ranging from a small handful to a few hundred different conditions (10).
It may seem more efficient to test for every genetic condition at once rather than screen for different genes in the various screening panels. However, genetic testing is complex and there are multiple types of sequencing methods that each have strengths and weaknesses for identifying different conditions. Genetic conditions are each caused by a different type of pathogenic change or changes, meaning that not every condition can be tested for in the exact same way. This makes testing for a large number of diseases with a single test more difficult and potentially less accurate than targeted testing (9).
Prenatal (Antenatal: During Pregnancy) Screening and Testing:
Prenatal screening and testing for genetic disorders during pregnancy was first introduced in the 1970s. It can be performed in the first, second or third trimester, with slight differences in method and accuracy between the trimesters (11). Historically, the process starts with a noninvasive screening test, usually a combination of an ultrasound measurement of the developing fetus and a blood test on the pregnant person to analyze biochemical markers. The screening can only determine if the fetus is at a higher risk for changes in the number of chromosomes, it cannot diagnose whether or not a genetic condition is present.
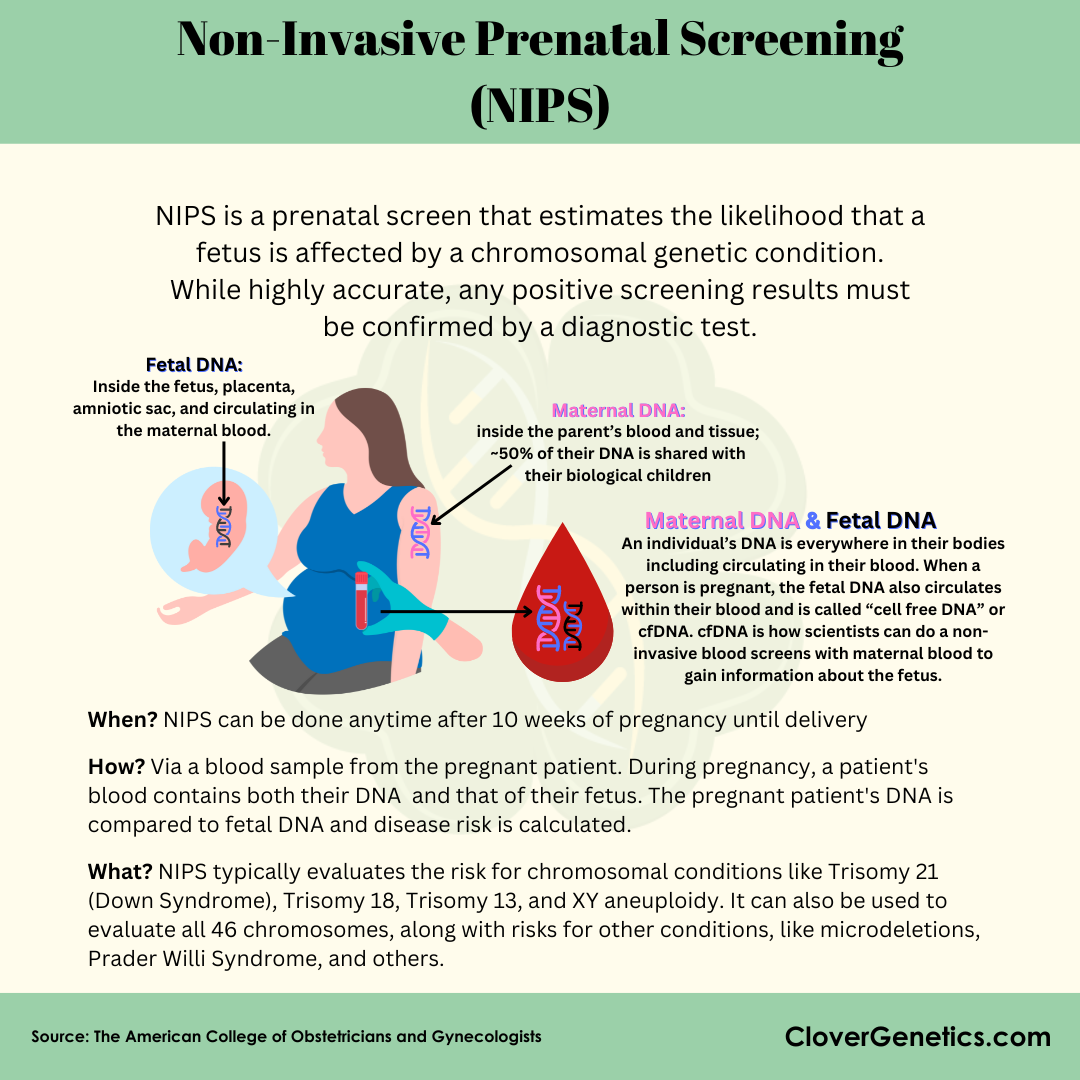
One such screening is called Non-Invasive Prenatal Screening (NIPS), became commercially available in 2011. This screening was formally called Non-Invasive Prenatal Testing (NIPT), but because it is not actually a test, they renamed it to reflect the actual type of analysis being performed. NIPS analyzes fragments of placental DNA that are found naturally in the blood of the pregnant person (12). NIPS has a much lower false positive rate than traditional serum screening, meaning that fewer individuals who are screened using this technique will need invasive diagnostic testing.
As of 2023, the ACMG strongly recommends NIPS over traditional screening methods to screen for fetal aneuploidies such as trisomies 13,18, and 21, which often go undetected when using alternative screening methods (15). Overall, modern NIPS is a promising technique for more accurate screening of genetic conditions. If an increased risk of a chromosomal abnormality was detected on a screening, the next step would be an invasive diagnostic test to confirm the presence of chromosomal differences (12).
The two most common prenatal diagnostic procedures are amniocentesis and chorionic villus sampling (CVS). In both techniques, DNA is extracted from these samples and tested to diagnose any unexpected variation in the fetus. Though amniocentesis and CVS are the current clinical standard for diagnosing fetal disorders, these diagnostic tests are associated with a slightly increased risk of miscarriage, between 1 in 100 to 1 in 500 (13). However, if NIPS was to come back positive, it may be recommended that the pregnant individual undergo amniocentesis or CVS to confirm the diagnosis, which is why the initial screening is almost always the first step (12).
Non-Invasive Prenatal Screening (NIPS)
Formerly called Non-Invasive Prenatal Testing, but since the blood sample is actually screening for disorders rather than directly testing, the update to the name of the screening was made to clarify the difference: Screenings are not as accurate as testing and is therefore not definitively positive until tested.
Amniotic Fluid & Amniocentesis
Amniotic fluid is a colorless, odorless liquid that surrounds and cushions a fetus during pregnancy, protecting them from infection and serving as a reservoir for fluid and nutrients from the parent (2).
Between 15-20 weeks of pregnancy, an amniocentesis can be performed to confirm the presence of genetic or chromosomal disorders (1). NIPS is generally performed prior to this invasive procedure. During amniocentesis a needle is used to penetrate through the abdomen and uterine wall into the amniotic sac, but not into the fetus itself, to take a sample of the amniotic fluid (13). From samples of amniotic fluid, a highly accurate genetic test is performed to confirm the presence of genetic or chromosomal disorders of the fetus. Amniotic fluid may also be analyzed to diagnose open neural tube defects (ONTD) such as Spina Bifida. It may also check for inherited gene defects and metabolic disorders (3).
This procedure is not without risk: pregnant people who have amniocentesis tests have an increased risk for miscarriage or pregnancy loss at a rate of approximately 1 in 100 patients undergoing the procedure.
**NOTE: Because of this, amniocentesis is only offered to pregnant people with a high chance of having a baby with a genetic condition. This is usually verified by family history for an inherited disease, previous pregnancies affected by a genetic condition, or a positive result on NIPS for Down Syndrome, Edwards’ Syndrome, Patau Syndrome, etc.**
How is it performed? The pregnant person lies flat on a medical bed and a numbing machine is pressed to her abdomen. The provider uses an ultrasound device to determine the location of the fetus and a thin needle is inserted into the abdomen to collect amniotic fluid (1).
What conditions CANNOT be identified through amniocentesis? Amniocentesis can only detect large chromosomal differences, or aneuploidies. Smaller genetic changes may not be detected (4).
Chorionic Villus Sampling (CVS)
During CVS, a needle or catheter is used to take a sample of placental cells from the same fertilized egg as the fetus (13).
**NOTE: Like amnio, CVS is an invasive test and comes with a risk for miscarriage (0.25 - 0.50%).**
Products of Conception
Product of Conception (POC) refers to tissue that has developed from the formation of an embryo, such as the placenta, chorionic villi, umbilical cord, or fetal tissue recovered after miscarriage, stillbirth, or abortion (5). For testing, fetal tissue or placental tissue is more informative than maternal tissue (decidua)(5).
What types of testing can be completed?
Example 1: After a miscarriage, genetic testing can be performed on fetal or placental tissue to investigate whether the pregnancy loss was a result of chromosomal aneuploidy (6).
Example 2: SNP microarray analysis can detect parental origin of chromosomal variation like triploidy, uniparental disomy, monosomy X, structural anomalies, or polyploidy (6). Learn more about aneuploidies here.
How is it generally performed? In a medical setting, POC tissue is removed from the lining of the uterus during a procedure called a D&C (dilation and curettage). The physician uses instruments or medication to dilate the cervix before utilizing sterile tools to remove uterine tissue. Samples must be stored in sterile tubes with tissue culture medium and antibiotics, then refrigerated or frozen if not used immediately (7).
What conditions CANNOT be identified through these samples? The usage of testing POC is typically limited to karyotyping, or the detection of structural aberrations or abnormal chromosome number. It usually is not used to test for smaller changes like substitutions or SNPs (6). Learn more about Karyotyping here.
Citations:
N.A. (n.d.). “Amniocentesis (amniotic fluid test).” Medline Plus. https://medlineplus.gov/lab-tests/amniocentesis-amniotic-fluid-test/
Fitzsimmons, E. D., and Bajaj, T. (2023, July 17). Embryology, Amniotic Fluid. Treasure Island, FL: StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK541089/
N.A. (n.d.). “Amniocentesis.” Johns Hopkins Medicine. https://www.hopkinsmedicine.org/health/treatment-tests-and-therapies/amniocentesis
N.A. (n.d.). “Chromosome Analysis, Amniotic Fluid.” Mayo Clinic Laboratories. https://genetics.testcatalog.org/show/CHRAF
Antonella, G., et al. (2015, October 1). Conservative and timely treatment in retained products of conception: a case report of placenta accreta retention. Int J Clin Exp Pathol 8(10): 13625-13629. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4680531/
Dahdouh, E. M. And Kutteh, W. H. (2021, March 22). Genetic testing of products of conception in recurrent pregnancy loss evaluation. Reproductive Biomedicine Online 43(1): 120-126. https://doi.org/10.1016/j.rbmo.2021.03.015
N.A. (n.d.). “Cytogenetics Products of Conception (POC) Chromosome Analysis.” Labcorp. https://womenshealth.labcorp.com/tests/180/cytogenetics-products-of-conception-poc-chromosome-analysis
The Trustees of the University of Pennsylvania. (n.d.). Carrier Screening. Pennmedicine.org. https://www.pennmedicine.org/for-patients-and-visitors/find-a-program-or-service/obstetrics/prenatal-genetic-testing/diagnosis-and-screening-services/carrier-screening
Gregg, A.R., Aarabi, M., Klugman, S. et al. Screening for autosomal recessive and X-linked conditions during pregnancy and preconception: a practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet Med 23, 1793–1806 (2021). https://doi.org/10.1038/s41436-021-01203-z
Romero, S., Rink, B., Biggio, J., & Saller, D. (2017). Committee opinion no. 690: Carrier screening in the age of genomic medicine. Obstetrics & Gynecology, 129(3). https://doi.org/10.1097/aog.0000000000001951 0
ACOG Committee on Practice Bulletins (2007). ACOG Practice Bulletin No. 77: screening for fetal chromosomal abnormalities. Obstetrics and gynecology, 109(1), 217–227. https://doi.org/10.1097/00006250-200701000-00054
Minear, M. A., Alessi, S., Allyse, M., Michie, M., & Chandrasekharan, S. (2015). Noninvasive prenatal genetic testing: Current and emerging ethical, legal, and Social Issues. Annual Review of Genomics and Human Genetics, 16(1), 369–398. https://doi.org/10.1146/annurev-genom-090314-050000
Centers for Disease Control and Prevention. (n.d.). Chorionic villus sampling and amniocentesis: Recommendations for prenatal counseling. Centers for Disease Control and Prevention. https://www.cdc.gov/mmwr/preview/mmwrhtml/00038393.htm
Prior, T. W., & Professional Practice and Guidelines Committee (2008). Carrier screening for spinal muscular atrophy. Genetics in medicine : official journal of the American College of Medical Genetics, 10(11), 840–842. https://doi.org/10.1097/GIM.0b013e318188d069
Dungan, J. S., Klugman, S., Darilek, S., Malinowski, J., Akkari, Y. M. N., Monaghan, K. G., Erwin, A., Best, R. G., & ACMG Board of Directors. Noninvasive prenatal screening (NIPS) for fetal chromosome abnormalities in a general-risk population: An evidence-based clinical guideline of the American College of Medical Genetics and Genomics (ACMG). Genetics in medicine : official journal of the American College of Medical Genetics, 25(2), 100336. https://doi.org/10.1016/j.gim.2022.11.004